By repeating the experiments made on first PMP with ZA4 PMP, we saw that some important PMP characteristics were changed, thus the embossing was no longer possible. So, another approach was tried. First, we tried to form a thick, ordered film of sub-micron spheres onto the membrane and we successfully performed this for a colloidal solution. By decreasing the colloid concentration, a compact and relatively (few layers) thin ordered film was formed (Figure 1 a, b).
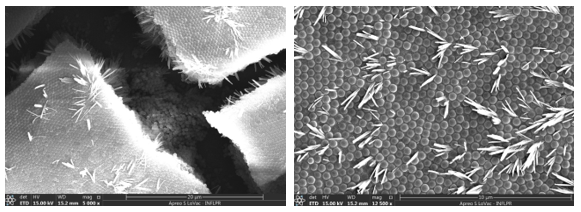
Fig. 1 Thin ordered films on ZA4 PMP (a on left, b on right).
By using a self-assembly method (under research), a micro-structured (Figure 2) single layer of SiO2 spheres was formed onto the ZA4 PMP’s surface.
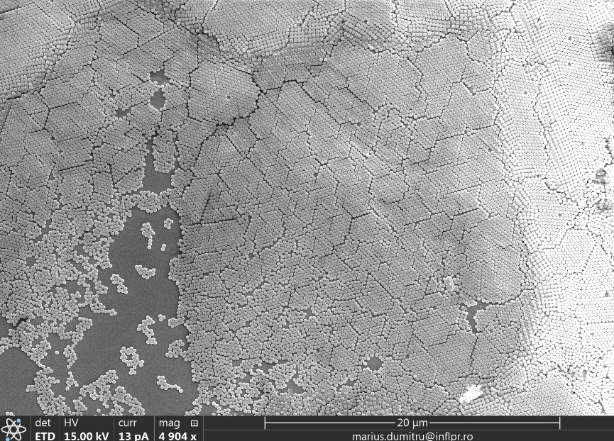
Fig. 2 Single layer of SiO2 spheres on ZA4 PMP.
However, because the colloid was a water based one, we observed that by water-membrane contact, a small fraction of the membrane surface dissolves, enter through the spheres’ holes, and re-solidify after water evaporation, forming an infiltrated opal like monolayer. We do not know if the dissolution of a superficial layer of polymeric membrane modifies its overall properties. If the answer would be positive, we could try to use a solvent which don’t produce the membrane dissolution.
The attempts for structuring the surface of polymeric membranes by self-assembling sub-micron sphere onto the polymeric surface have remarqued the different behavior of the three kinds of PMP’s to the temperature and moisture (water) action (Figure 3).
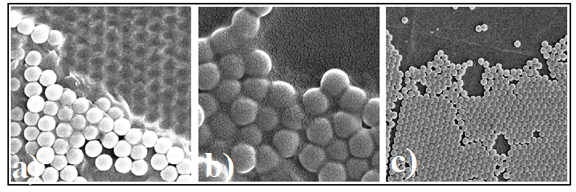
Fig. 3 Sub-micron sphere monolayers self-assembled onto polymeric membranes surface: a) PMP 1, b) PMP ZA4, c) PMP ZB4.
However, if on PMP 1 only thermo-mechanical treatments could be performed, on the ZA4 and ZB4 the effect of water to their surfaces was studied. If PMP 1, seems to present a thermo-plastic behavior allowing an embossing process, ZA4 and ZB4 PMPs seems to be thermoset materials. Also, while ZA4 is a swelling PMP, ZB4 stays against the water. This allowed us to deposit and form by self-assembling a sub-micron sphere single layer onto the ZB4 PMP’s surface (Figure 4). The thermo-mechanical action didn’t change (apparently) the ZB4’s structure.
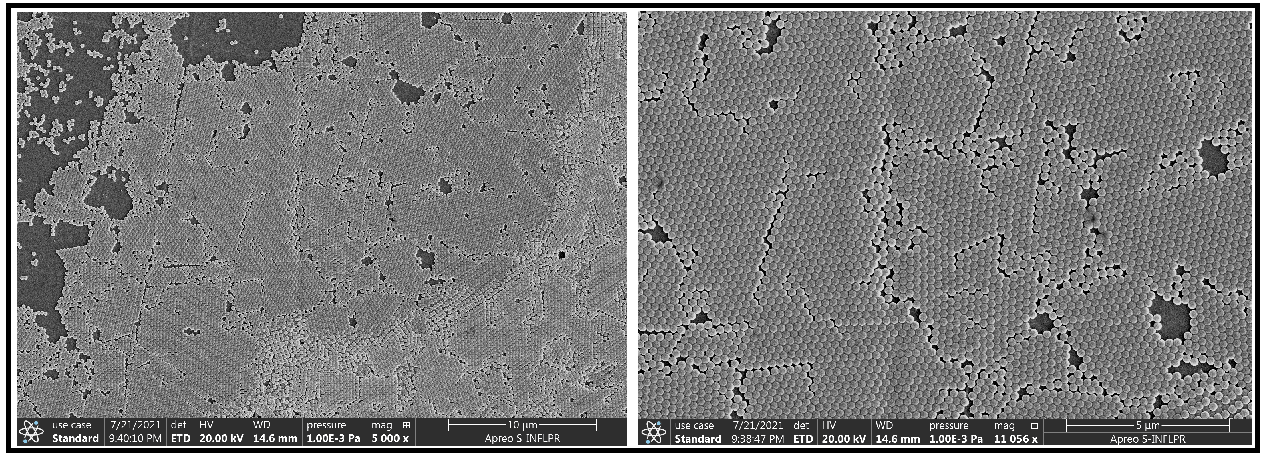
Fig. 4 SEM images of a single-layer of 250 nm silica spheres onto ZB4 polymeric membrane.
The large surface of spheres single layer which were formed suggest also an accentuated hydrophilic character of ZB4 PMP. However, for continuing the investigations we must know how ZB4 PMP reacts to acetone or toluene action.


